Lotus Clinical Research 2019-2020 Highlights
Baudax Bio Non-Opioid Pain Drug Approval:
In February, FDA granted approval to longtime Lotus client/ collaborator Baudax Bio’s non-opioid postsurgical pain therapy ANJESO. Lotus designed and performed both the Phase 2 and Phase 3 programs for this drug, with extensive scientific and operational input at every stage. The Baudax bunionectomy and abdominoplasty pivotal surgical studies that led to this approval were the first Phase 3 pivotal clinical trials that Lotus performed as both a contract research organization (CRO) and site.
The drug is a long-acting non-steroidal anti-inflammatory drug (NSAID), which will allow patients to be treated with a single NSAID dose after outpatient surgery and experience 24 hours of analgesic relief. This could help significantly reduce the number of patients needing postoperative opioid prescriptions, which is a step forward for Lotus’ mission and vision of reducing the dispersion of opioids into the community.

Continuing Work to Mitigate the US Opioid Crisis:
Lotus continues work on clinical trials for multiple non-opioid pain therapies as well as less harmful/ less addictive opioid analgesics. We’re working on several important clinical trials in postoperative pain as COVID-19 restrictions on elective surgeries have been lifted.
COVID-19 Clinical Trials:
Lotus is in the early stages of designing and performing clinical trials on multiple promising therapies related to COVID-19. Drug developers with potential therapies are relying on Lotus’ capabilities to fast track development programs that could potentially lead to a reduction in COVID-19 illnesses and fatalities. The details of these early-stage projects are still confidential, but we are prioritizing this new therapeutic area and are proud to be part of the effort to fight COVID-19.
Recent Lotus Scientific Publications:
In the past 12 months, our Chief Scientific Officer Dr. Neil Singla has co-authored 3 published scientific manuscripts on non-opioid or less harmful opioid pain treatments, as well as co-authoring the IMMPACT group’s recommendations on improving study conduct and data quality in clinical trials of chronic pain treatments. IMMPACT is a public-private partnership drawn from academia, regulatory agencies (US FDA, European Medicines Agency), US National Institutes of Health, US Veterans Administration, consumer support and advocacy groups and industry.
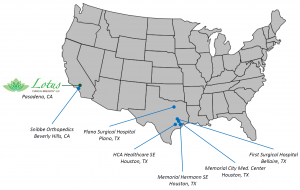
Site Network Partnership:
This past September, Lotus partnered with Texas-based HD Research to form a network of specialized clinical trial sites with the aim of optimizing and standardizing the conduct of clinical trials for pain therapies. Lotus will communicate the best practices we’ve learned to clinical research centers across the US to help the pain research community perform safer and more accurate clinical trials.