High-quality, protocol-specific analyses.
Study Design.
More and more, sponsors are recognizing that adaptive designs can allow them to save both time and money in the drug development process, but there are many considerations that should be reviewed prior to jumping in. At Lotus Clinical Research, we are able to explain the pros and cons of various approaches with our clients in order to help them to make the best decisions.
We have processes in place to enable Lotus to provide both blinded and unblinded support for a study. This is important for both supported oversight committees like DSMBs or SRCs. It also allows us to run and validate complex modeling analysis during a study, which ensures we are ready to deliver topline results within days of a database lock.
Lotus Clinical Research can support a randomization strategy that best aligns with a study’s goal. Our biostatisticians have experience from simple 1:1 ratio for a single site study, to a more complex adaptive randomization scheme across multiple sites.
We review prior studies and literature in the indication to help our sponsors select the most appropriate, scientifically relevant endpoints for their studies. The choice of endpoint, (both primary and any key secondary) is key in supporting the objectives of the trial and the sponsor company.
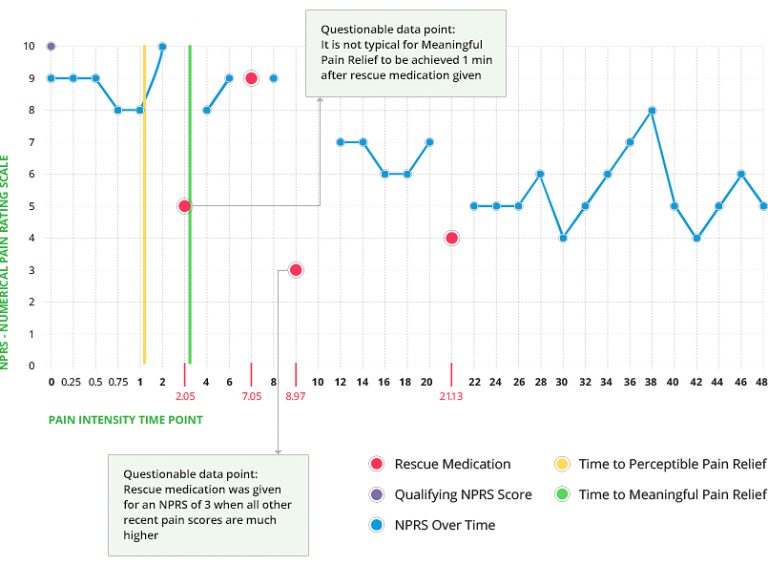
NPRS Over Time
Home » CRO Services » Biostatistics
Biostatistics
An experienced biostatistician will be assigned to each study to provide high-quality, protocol-specific analyses with timeliness, dependability, and audit-ready documentation. Lotus’ proposed statistical analysis will be based specifically on the complexities of the trials and the anticipated statistical analysis scope of work. The Biostatistics team will develop the randomization plan; generate the Statistical Analysis Plan (SAP), including development of mock tables, listings and figures (TLFs); manage all programming activities; perform the statistical analyses outlined in the protocol; and collaborate with medical writers to ensure overall accuracy of the final study report. The biostatistics project team will also assist with the development of the CRF. The lead biostatistician will work closely with other team members, including clinical, data management, and medical writing to support and resolve any statistical related issue affecting the scientific integrity of the study.
Study Design
Making sure that the statistical foundation of your clinical trial is solid ensures the integrity of your trial, helps reduce costs, and accelerates results. Our experienced biostatisticians will look at the latest literature and most recent regulatory guidance to suggest the study design that best supports your objectives. Aspects we will consider include:
Sample Size Determination
Our statisticians will help to determine the most appropriate sample size to support your results. We help your team appropriately incorporate historical information and make sure to keep the key study objectives in mind so they can make informed decisions about the assumptions used to design and power their clinical trials.
Adaptive Designs
More and more, sponsors are recognizing that adaptive designs can allow them to save both time and money in the drug development process, but there are many considerations that should be reviewed prior to jumping in. At Lotus Clinical Research, we are able to explain the pros and cons of various approaches with our clients in order to help them to make the best decisions.
Blinding
We have processes in place to enable Lotus to provide both blinded and unblinded support for a study. This is important for both supported oversight committees like DSMBs or SRCs. It also allows us to run and validate complex modeling analysis during a study, which ensures we are ready to deliver topline results within days of a database lock.
Randomization Strategies
Lotus Clinical Research can support a randomization strategy that best aligns with a study’s goal. Our biostatisticians have experience from simple 1:1 ratio for a single site study, to a more complex adaptive randomization scheme across multiple sites.
Endpoint Selection
We review prior studies and literature in the indication to help our sponsors select the most appropriate, scientifically relevant endpoints for their studies. The choice of endpoint, (both primary and any key secondary) is key in supporting the objectives of the trial and the sponsor company.
Patient Profile Creation
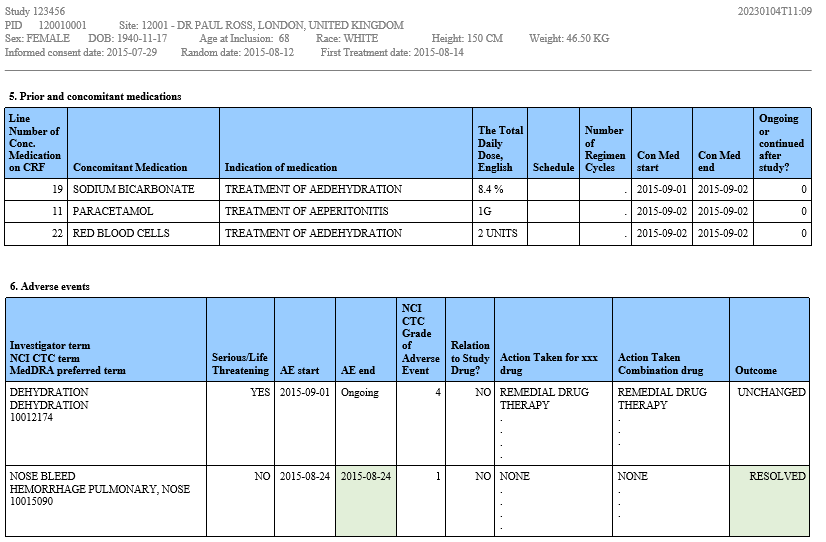
Just as critical as the analysis of study results is the ability to provide tools to support proper oversight. Our Programming team has experience creating both table and graphical patient profiles. In either case the critical data is presented in a concise format for each patient. The below example shows two of more than 12 tables that were created for the medical monitor. The color coding on some of the tables indicates data that has been updated since the last review.